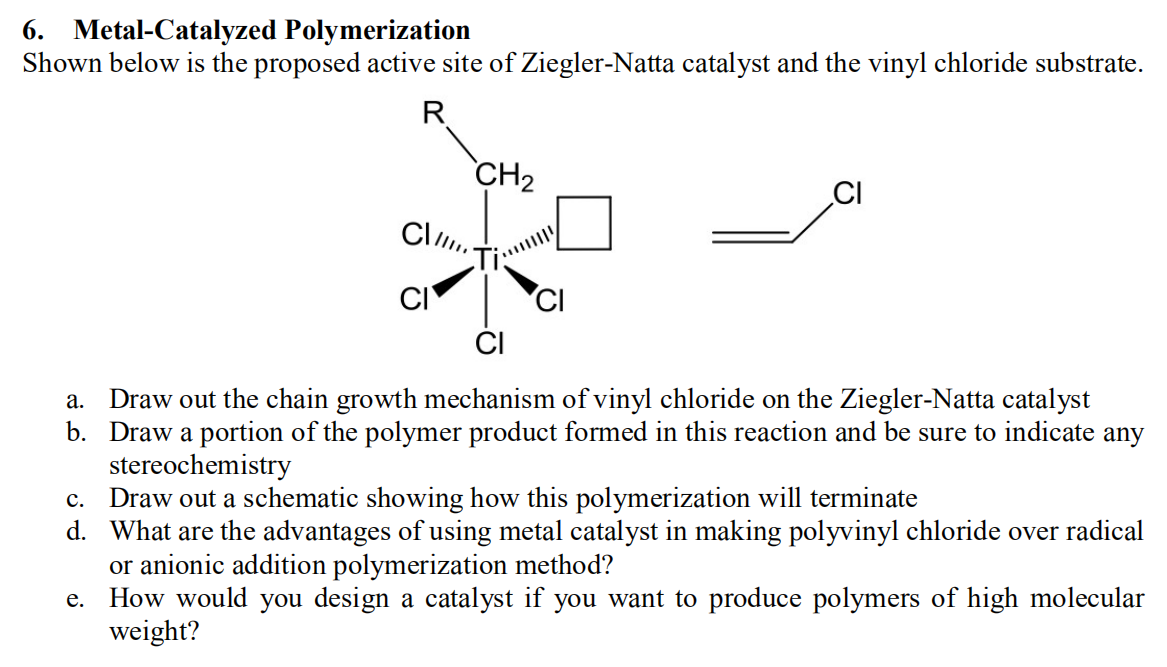
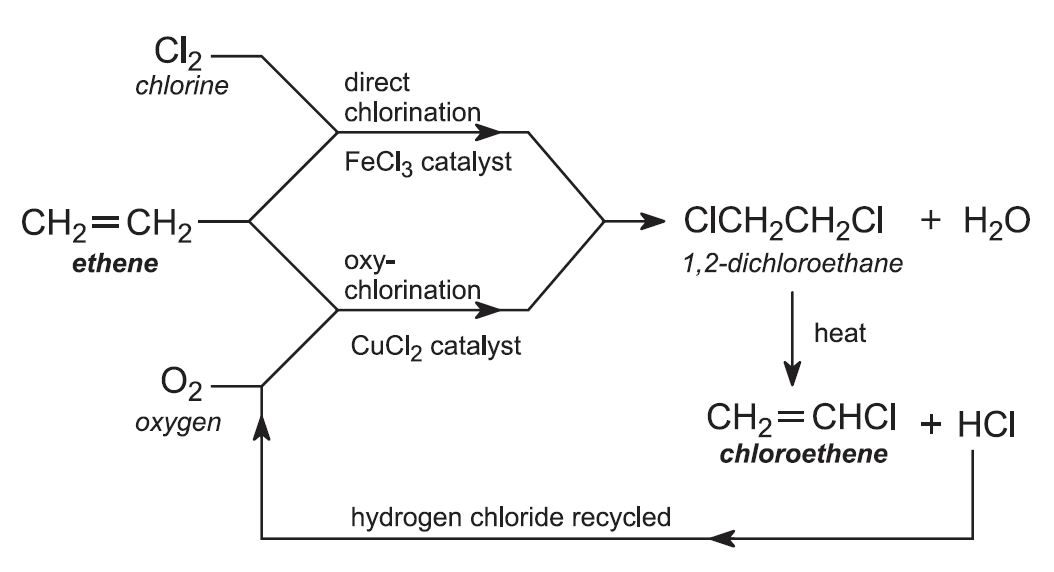
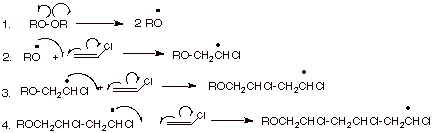
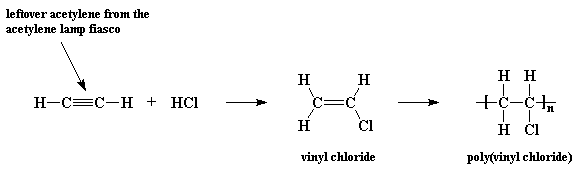
The mechanism of free-radical polymerization of vinyl chloride monomer... | Download Scientific Diagram

what are the different ways of initiating addition polymerisation describe one of them for polymerising vinyl chloride - Chemistry - Polymers - 7023843 | Meritnation.com

Modeling of Suspension Vinyl Chloride Polymerization: From Kinetics to Particle Size Distribution and PVC Grain Morphology | SpringerLink
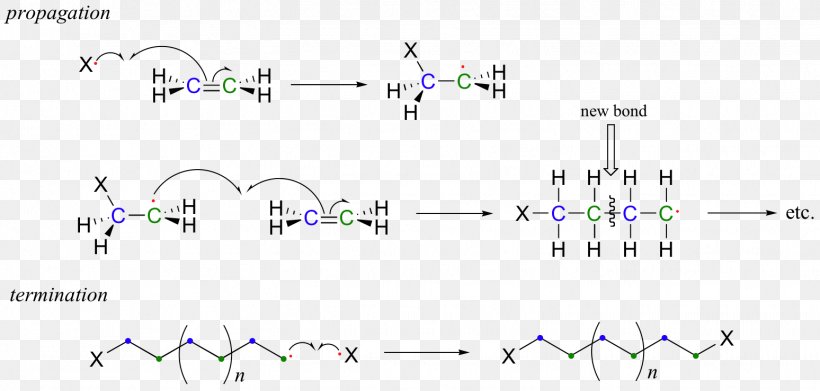
Ethylene Radical Polymerization Radical Polymerization Polyvinyl Chloride, PNG, 1548x740px, Ethylene, Addition Reaction, Alkene, Body Jewelry, Chain
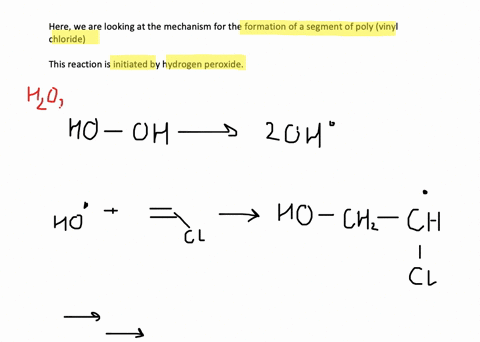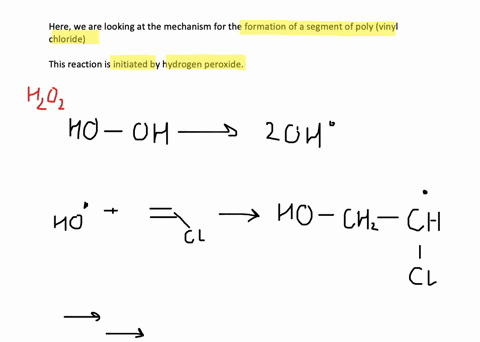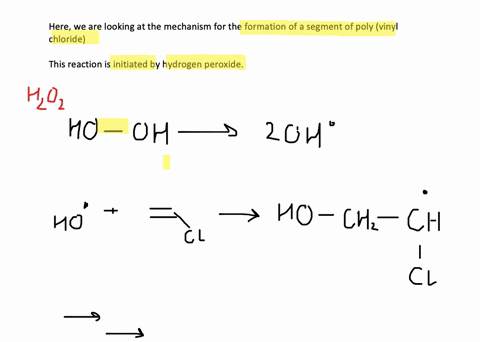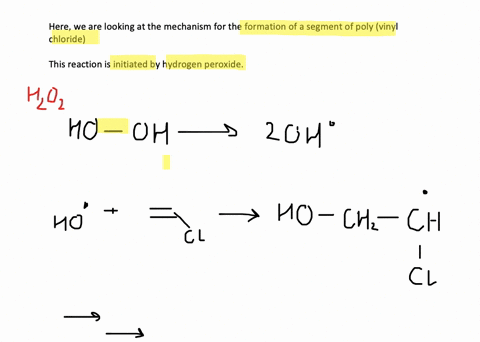
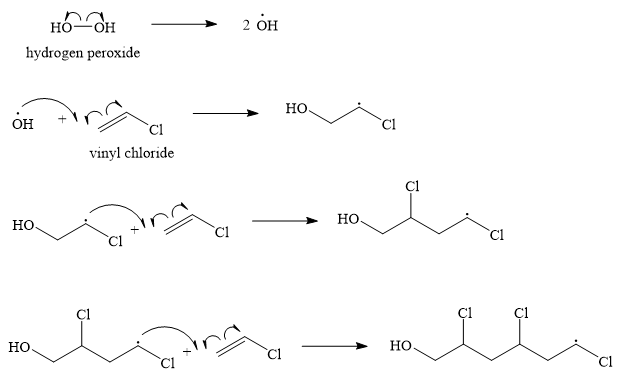
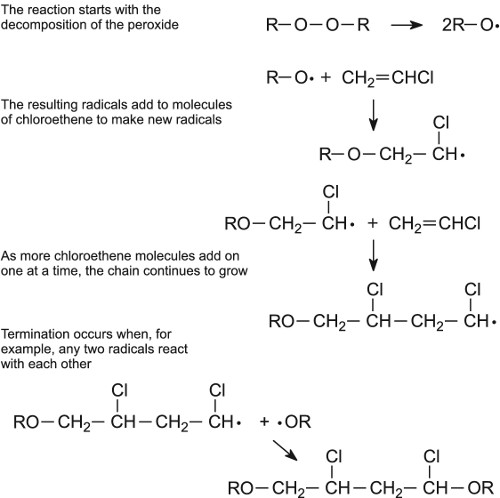
SOLVED:Show the mechanism for the formation of a segment of poly(vinyl chloride) that contains three units of vinyl chloride and is initiated by hydrogen peroxide.

Copolymerization Studies of Vinyl Chloride and Vinyl Acetate with Ethylene Using a Transition-Metal Catalyst | Journal of the American Chemical Society

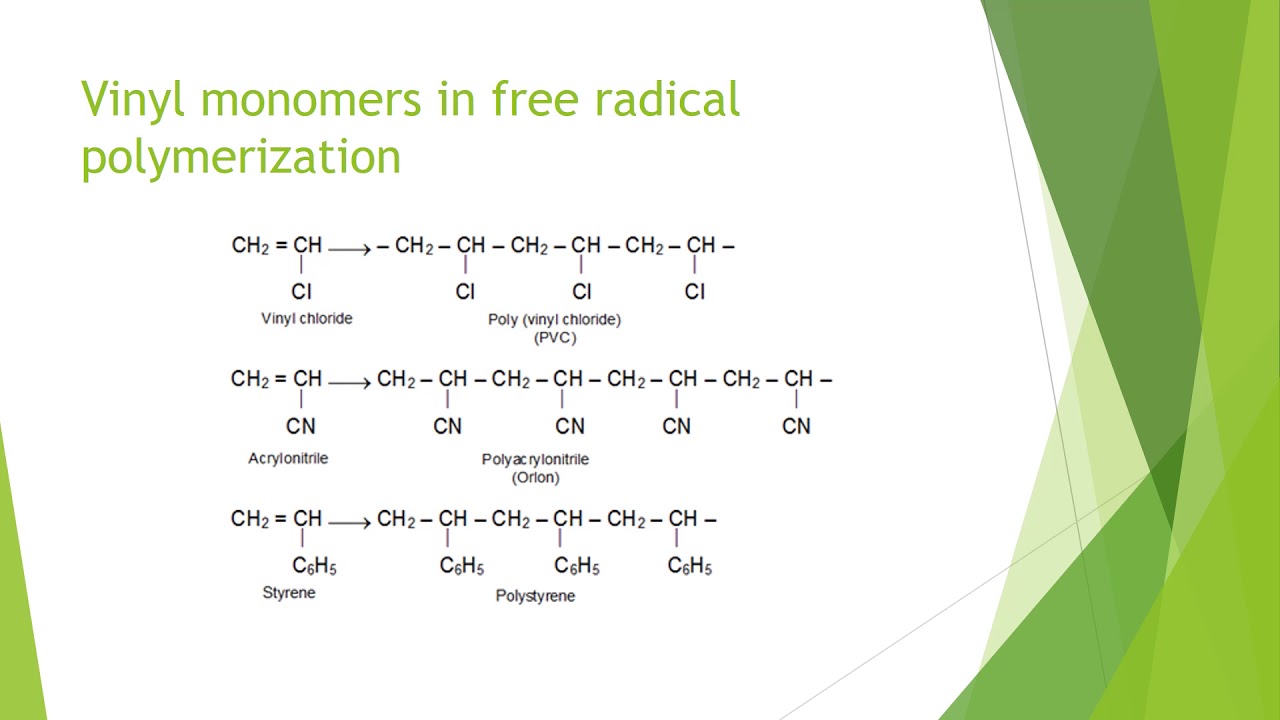
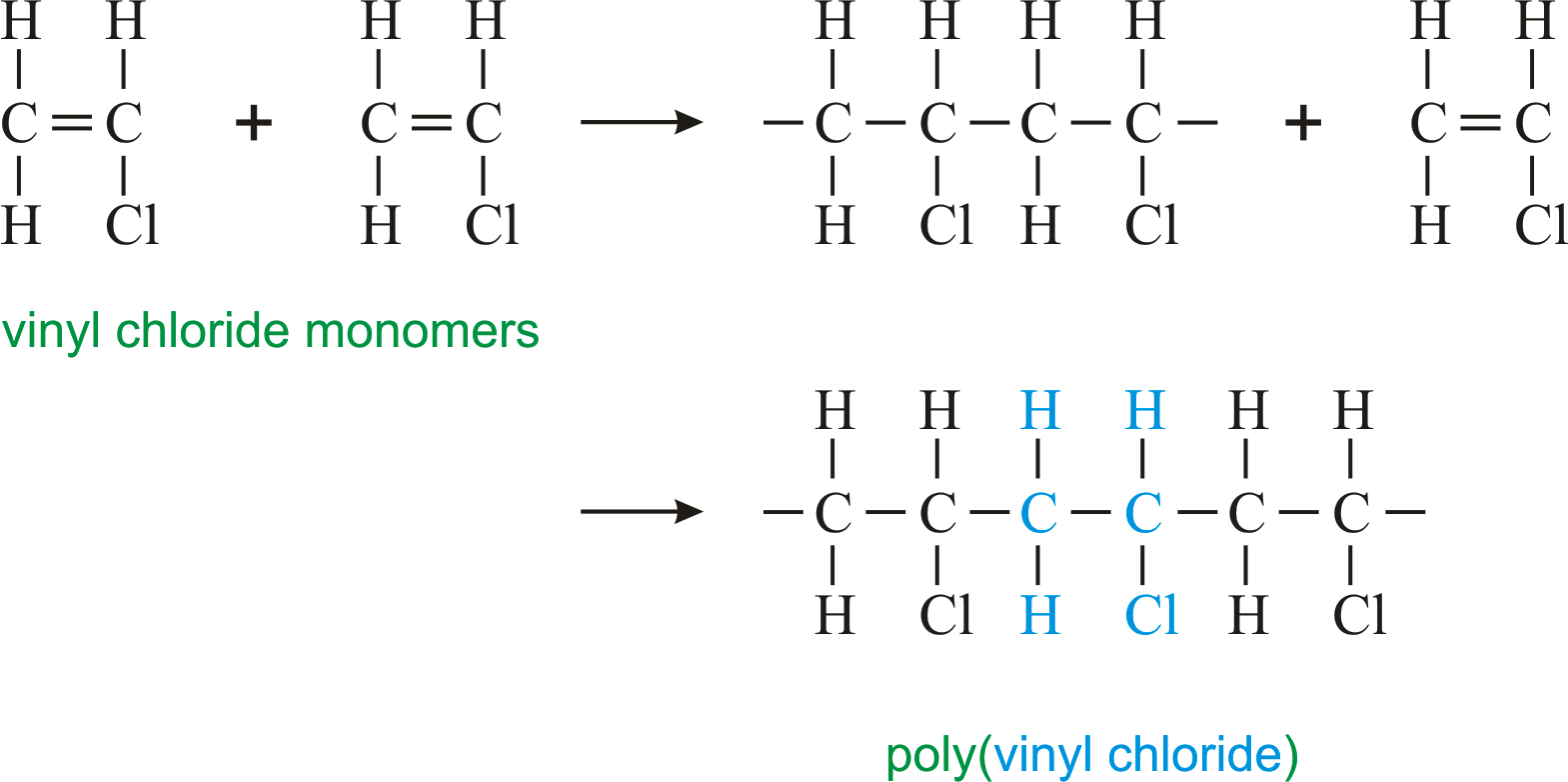
Poly(vinyl chloride) (PVC) is an addition polymer of vinyl chloride C2H3Cl. Write the equation for the formation of the polymer. | Homework.Study.com

All-PVC” Flexible Poly(vinyl Chloride): Nonmigratory Star-Poly(vinyl Chloride) as Plasticizers for PVC by RAFT Polymerization | Macromolecules